Emphysematous pyelonephritis (EPN) is an uncommon destructive gas-producing infection of the renal parenchyma and collecting system [
1]. It most commonly occurs as a complication of uncontrolled diabetes mellitus, followed by urinary tract obstruction in 25-40% of human cases.
Escherichia coli is the most common cause, seen in nearly 70% in human medicine [
2]. There are 2 types of EPN in humans. Type 1 EPN is defined as destruction and/or fluid retention in renal parenchyma. Whereas, type 2 is characterized by the fluid accumulation in the renal or perirenal parts and gas production in the collecting system [
3]. Type 1 EPN has a poor prognosis compare to type 2. Although EPN has not been classified in the veterinary literature, its cases have been reported, including 2, 1, and 1 case associated with diabetes, urinary tract infection, and a portosystemic shunt in a dog with high mortality, respectively [
3,
4]. Herein, we report a case of EPN associated with ureteral obstruction by calcium oxalate uroliths in a dog.
An 11-year-old intact female mixed-breed dog was referred because of lethargy, anorexia, and vomiting. On physical examination, no significant abnormality was found. Complete blood count showed mild leukocytosis (20.75 ├Ś 10
9/L) and thrombocytopenia (134 ├Ś 10
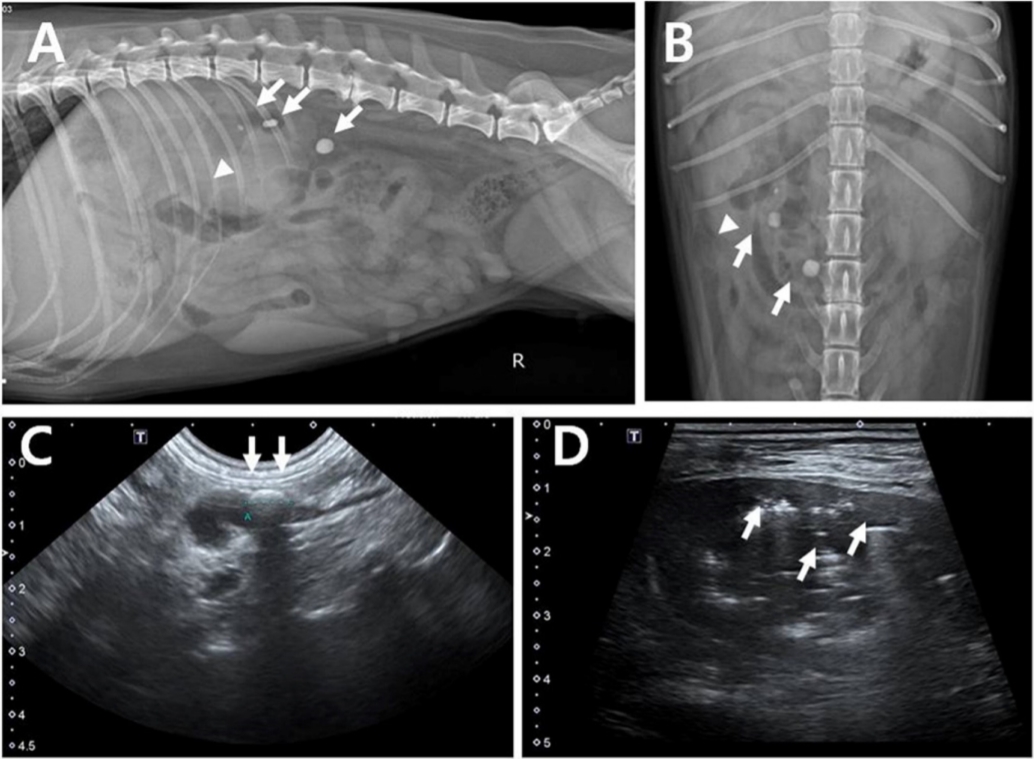
9/L). Serum chemistry revealed an elevated alkaline phosphate level (749 U/L). Serum urea nitrogen (48 mg/dL) and creatinine (1.6 mg/dL) were unremarkable. The dipstick test revealed proteinuria (30 mg/dL), slight aciduria (pH: 5), and normal urinary specific gravity (1.023). Urine cytology revealed rod-shaped bacteria and neutrophils. On radiography, the right kidney was enlarged, with snowman-shaped gas opacity, and 3 radiopaque ureteral uroliths were present in the proximal ureter. The renal pelvis was dilated by echogenic fluid with gas containing a urolith, 7.6 mm in diameter, with shadowing in the proximal ureter on ultrasonography (Aplio 400; Toshiba Medical Systems Corp., Japan) (
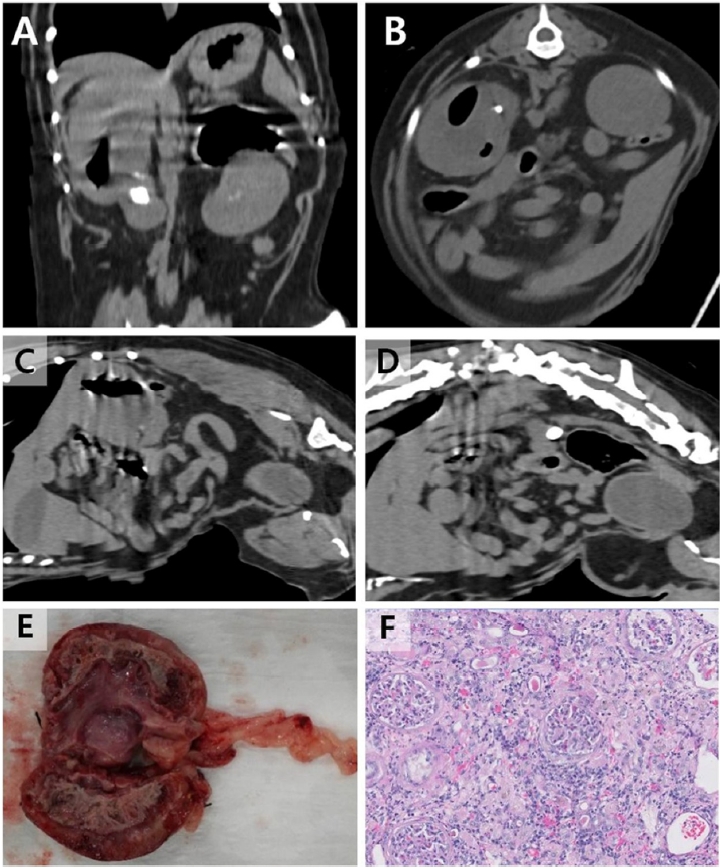
Fig. 1). Due to the poor body condition, computed tomography (CT, Alexion Advance; Toshiba Medical Systems Corp.) was performed in the awake dog without anesthesia or sedation in sternal recumbency in a cat restraint bag. In the right kidney, non-enhancing gas material was detected. Three uroliths were found in the right proximal ureter. Based on these results, type 1-like EPN with ureteral uroliths was diagnosed (
Fig. 2).
For the right ureteronephrectomy, the renal vessels and proximal ureter were ligated with a size 0 silk suture (Black silk, AILEE Corp., Korea) and resected. Abdominal lavage was performed with sterile warm saline before closure. The purulent contents of the removed kidney and ureter were used for bacterial isolation and histological evaluation. Ureteral uroliths were analyzed for the composition of the stones. The resected kidney revealed that the renal pelvis and parenchyma were filled with copious brown pus. During hospitalization, Ampicillin (22 mg/kg intravenous [IV] every 8 h [q8h], Penbrex inj.; Yungjin, Korea), Enrofloxacin (5 mg/kg IV q24h, Baytril inj.; Byer Korea, Korea), Famotidine (0.5 mg/kg IV q12h, Gaster inj.; Dong-A Pharm, Korea), Butorphanol (0.01 mg/kg IV q8h, Buthophan inj.; Myungmoon Pharm, Korea), and Lactated ringer solution (10 mL/kg IV q1h; CJ Health care, Korea) were administered. The patient recovered after the surgery without any complication and was discharged on postoperative day 3. E. coli was isolated from the pus, and it was sensitive to ampicillin and enrofloxacin. The stone was consisted of 80% calcium oxalate monohydrate and 20% magnesium ammonium phosphate (struvite). Histological findings confirmed severe pyelonephritis effacement of almost the entire renal architecture that could be significantly compromising renal function. The ureter had severe inflammation with focal ulceration. At the 6-month follow-up, the clinical signs had improved, and no complications were observed.
EPN is an uncommon condition in both humans and animals. The most common cause of EPN is diabetes mellitus with excretion of excess glucose in the urine and infection by gas-producing bacteria. Ureteral reflux induced by upper urinary tract obstruction could also cause EPN [
3]. There are 2 reports of gas formation in the urinary tract associated with diabetes mellitus in dogs whose prognosis was guarded because of emphysematous pyelitis and cystitis during the medical treatment [
5,
6]. In addition, in one dog with a congenital portosystemic shunt disease, ammonium urate ureteral stone caused ureteral obstruction and resulted in pyelonephrosis associated with
E. coli infection [
7]. Common bacterial causes of EPN include
E. coli,
Klebsiella species,
Aerobacter spp., and
Proteus species in humans [
2,
3,
8,
9]. In our case,
E. coli was also isolated in the purulent exudate in the resected kidney.
This is the first report of type 1 EPN due to calcium oxalate uroliths of the upper urinary tract of a dog. Ureteral urine reflux to the pelvis might cause emphysematous infection of the kidney. In this case, pus and gas were localized in the renal parenchyma, pelvis, and ureter but not in the bladder. We suppose that although the dog was female, calcium oxalate uroliths had formed first because of the acidity of urine, and struvite was secondarily involved by urinary tract infection.
To diagnose EPN, ultrasonography had been commonly used in veterinary patients. In our case, multiple bright echogenicities associated with acoustic shadowing in the renal pelvis and ureter parts were seen, similar to human cases of EPN [
3,
4,
10]. The dirty shadow artifact was characterized by a chaotic display of continuous echoes throughout the field. CT is the most specific diagnostic method for monitoring gas-containing urinary tract infections in humans [
4,
6]. CT is superior for excluding complex lesion of emphysematous pyelitis including renal fluid accumulation, abscesses, or EPN. In this report, we performed CT in an awake patient because of the poor body condition and the owner's hesitance to general anesthesia. This is the first time CT was performed in an awake dog to diagnose EPN. In our case, CT revealed gas contents in the renal parenchyma with little deterioration of image quality due to slight motion in the awake patient. Previous reports have also demonstrated CT in awake or sedated dogs with satisfactory image quality [
10,
11]. For the definitive diagnosis of upper airway obstruction on brachycephalic dogs, CT and 3-dimensional rendered endoscopy were performed without general anesthesia [
10]. Another report demonstrated that contrast-enhanced multidetector CT was useful to detect pulmonary thromboembolism with pyothorax in an awake dog [
12]. In this case, we did not performed dynamic CT using iodinated contrast media to prevent the renal injury. Moreover, we were worried about the excessive movement of the dog due to the burning sensation when contrast medium was injected. Therefore, we recommend CT in awake dogs that are obedient and have a high risk of anesthetic complications for the definite diagnosis of critical diseases that do not essentially need dynamic CT, such as EPN, laryngeal or tracheal airway obstruction, pulmonary thromboembolism, etc.
In our case, we performed ureteronephrectomy as a treatment option because histopathological findings showed that most of the renal medullary structures were destroyed because of severe inflammation. Preserving the damaged kidney using insertional ureteral stents or ureterotomy to remove only the ureteral stones have been attempted. However, the results show poor prognosis and high possibility of sepsis [
13]. In the cat, a 2.5-Fr, 14-cm ureteral stent was inserted through the bladder and proximal ureter for the management of EPN; however, after 9 postoperative months, nephrectomy was performed because of the recurrence of hydronephrosis [
13]. In humans, traditional ureteronephrectomy is also recommended in type 1 EPN, although ultrasound-guided percutaneous drainage catheters are used for the treatment of type 2 EPN [
1,
9,
14]. Therefore, in cases of type 1 EPN with severe inflammation, aggressive surgical resection is appropriate for veterinary patients.
In conclusion, this was the first report of type 1 EPN associated with calcium oxalate uroliths. CT in the awake status was effective in reducing the risks of complications of general anesthesia and in evaluating the disease condition accurately. In addition, we recommend ureteronephrectomy in dogs with type 1 EPN when the contralateral kidney is intact in function for rapid recovery and good prognosis. Further studies are necessary to confirm the effectiveness of other non-surgical alternatives for dogs with type 2 EPN.









 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print