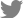Getah virus (GETV), an arthropod-borne virus transmitted by mosquitoes, belongs to the genus
Alphavirus in the family
Togaviridae [
1]. The
Alphavirus genome consists of a linear, positive-sense, single-stranded RNA approximately 11 kb in length. This genome is composed of a 5' terminal cap, four nonstructural protein genes (nsP1 to nsP4), five structural protein genes (C, E3, E2, 6K, and E1 protein), and a 3' poly A tail. GETV belongs to the Semliki Forest antigenic complex, along with Bebaru virus, Chikungunya virus (CHIKV), Mayaro virus, O'nyong-nyong viruses (ONNV), Ross River virus (RRV), Semliki Forest virus (SFV), and Una virus [
2].
GETV was first isolated in 1955 from mosquitoes (
Culex gelidus) in Malaysia [
3]. Although Sagiyama virus (SAGV) was also isolated from the mosquito
Culex tritaeniorhynchus at a similar time, SAGV is now regarded as a strain of GETV [
4]. GETVs can be grouped into four evolutionary groups [
5], with Group I including the prototype of GETV (MM2021); Group II including the SAGV strain; Group III, the most abundant group, being associated with several epidemic outbreaks of GETV; and Group IV being relatively restricted [
6-
8].
The transmission cycle of GETV includes horses and pigs as major amplifiers and mosquitoes as the primary arthropod. Clinical indications of GETV infection in horses include fever, anorexia, edema of the hind limbs, enlargement of lymph nodes and rash [
9]. In pigs, GETV infection is associated with depression, tremors, hind limb paralysis, diarrhea, and high mortality rates in piglets, and reproductive failure in pregnant sows that leads to stillbirth and fetal death [
10,
11]. GETV is responsible for infection in a broad range of animals including cattle, foxes, and wild boars [
12-
14]. Neutralizing antibodies have been detected in humans, but GETV infection is considered subclinical.
Several outbreaks of GETV have been reported in horses and pigs, causing large economic losses [
9,
10]. In particular, a GETV outbreak in China in 2017 caused the deaths of approximately 200 piglets and stillbirths in more than 150 pregnant sows [
11]. Because GETV is mainly transmitted by mosquitoes and China is adjacent to South Korea, it is important to identify and prepare for the risk of GETV outbreaks in South Korea. Although several studies have assessed the seroprevalence of GETV in racehorses in South Korea [
15], no study to our knowledge has reported the seroprevalence of GETV in pigs in South Korea. The present study therefore utilized a virus neutralization (VN) test to evaluate the seroprevalence of GETV in South Korean pigs.
A total of 670 whole blood samples were collected from pigs at pig farms and slaughterhouses in eight provinces and one city throughout Korea, including 67, 40, 113, 23, 192, 158, 20, and 20 samples from Jeollanam-do, Jeollabuk-do, Gyeonggi, Gyeongsangbuk-do, Gyeongsangnam-do, Jeju, Gangwon, and Chungcheongbuk-do provinces, respectively, and 37 from Seoul. Of the 670 blood samples, 291 (43.4%) were from sows and 379 (56.6%) were from fattening pigs. Whole blood samples were centrifuged at 2,500 × g for 10 minutes at 4°C to collect sera. The serum samples were heated at 56°C for 30 minutes to inactivate serum complement and stored at -20°C until use.
Vero cells (ATCC CCL-81; ATCC, USA) were maintained in Dulbecco Modified Eagle medium (DMEM; Gibco BRL, USA) containing 10% fetal bovine serum (Gibco BRL), penicillin (100 units/mL), streptomycin (100 units/mL), and amphotericin B (0.25 µg/mL) at 37℃ in a 5% CO2 incubator. The QIAG9301 strain (accession number: KR081238) of GETV used for VN tests had been isolated in 1993 from the whole blood of a pig in South Korea. The QIAG9301 strain was prepared after evaluating its titer using the Reed-Muench method. Briefly, 100 µL of 10-fold serial dilutions of the virus were mixed with the same volume of Vero cells (2 × 105 cells/mL) in DMEM containing 10% fetal bovine serum in each well of a 96-well plate. The plates were incubated at 37°C for 3 to 5 days until cytopathic effects were observed.
VN tests were performed using the microneutralization test technique. Briefly, 50 µL aliquots, of 2-fold serial dilutions (1:2 to 1:256) of heat-inactivated sera in DMEM were mixed with an equal volume of 200 TCID50/0.1 mL in each well of a 96-well plate. After incubation for 1 hour at 37°C, 100 µL of a Vero cell suspension containing 2 × 105 cells/mL was added to each well. Uninfected cell cultures were used as negative cell controls. The 96-well plates were incubated for 5 days at 37°C in an atmosphere containing 5% CO2. The antibody titers were expressed as the reciprocal of the highest serum dilution that 100% inhibited cytopathic effect. Antibody titers ≥ 1:2 were considered positive.
The overall seropositive rate of GETV in domestic pigs in South Korea was 26.4% (177/670) (
Table 1), higher than the seropositive rates of GETV in racehorses of 12.4% in 2013 and 12.2% in 2014 [
15]. Because a vaccination program against GETV infection has not yet been implemented in South Korea, these findings suggest that all antibodies were induced by GETV infection.
The seropositive rates varied widely by region of sample collection, with these rates ranging from 7.5% to 70.3% (
Fig. 1). Seoul had the highest rate of seropositivity, 70.3% (26/37), followed in order by Chungcheongbuk-do (70.0%, 14/20), Gangwon (50.0%, 10/20), Gyeongsangbuk-do (47.8%, 11/23), Jeollabuk-do (35.0%, 14/40), Jeju (32.9%, 52/158), Gyeonggi (21.2%; 24/113), Gyeongsangnam-do (10.9%, 21/192), and Jeollanam-do (7.5%, 5/67) provinces. By contrast, seropositive rates were similar in sows (24.1%, 70/291) and fattening pigs (28.2%, 107/379), as were antibody levels (
Table 1). Even within individual provinces, there were noticeable differences in seropositivity rates among farms (data not shown). Given that GETV is transmitted by mosquitoes, the housing environment of farms or the timing of each sample collected were thought to have affected these results.
The levels of viral neutralizing antibody generally ranged from 1:2 to 1:16, with 63.3% (112/177) of the positive samples having titers of 1:2 (
Table 1). These low level of VN titers could be affected by a seasonal factor, such as the timing of sample collection. Alternatively, considered that the GETV QIAG9301 strain was isolated from the pig serum collected in 1993, the low similarity with the recently circulated GETV is thought to impact the level of VN titer. Despite these low levels of GETV neutralizing antibody, the overall seropositive rate has increased over 7 years. Because global warming can affect the prevalence of vector-borne diseases, including GETV, novel epidemics may emerge, such as the recent GETV outbreak in China [
11]. Periodic serological monitoring of mosquito-related diseases, as well as mosquito eradication programs, are necessary to reduce the risk of outbreak of these diseases. Because horses and pigs are considered amplifying hosts in the GETV transmission cycle, it is necessary to regularly monitor the seroprevalence of anti-GETV antibodies in these amplifying hosts. Because mosquitoes are a major vector for this disease, mosquitoes should be regularly monitored to prepare for emerging risks.
In conclusion, this serological survey of GETV in South Korean pigs evaluated the presence of neutralizing antibodies in 670 whole blood samples collected from domestic pigs in eight provinces and one city throughout South Korea. The overall seropositive rate was 26.4%, higher than the rates in racehorses in 2013-2014. Regional differences in seropositivity rates were observed. These rates ranged from 7.5% to 70.3%, suggesting that the environment of farms housing these animals may be the most critical factor associated with seropositivity. The findings of this study also indicate that VN tests can be used to monitor anti-GETV antibodies in pig serum samples. Preparations for epidemics of novel diseases caused by climate change should include regular sero-surveillance for these diseases, including GETV, and the development of vaccines against these novel pathogens.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print