Equine piroplasmosis (EP) is a tick-borne protozoal disease in which horses, mules, donkeys, and zebras are infected with the hemoprotozoan parasites,
Babesia caballi or
Theileria equi from blood-sucking ticks [
1,
2]. EP causes serious economic damages, showing hemolytic anemia and associated systemic illness [
1]. Furthermore, EP has been an obstacle to international trade as the disease designated by World Organization for Animal Health [
3-
5]. EP is an endemic disease prevalent in the tropical and subtropical regions and in some temperate regions [
1,
2]. It has predominantly been reported in Asia, South and Central America, Africa, Southern Europe, and some parts of southern USA [
1,
2].
EP antigen diagnosis is performed by microscopic examination and polymerase chain reaction (PCR), and the antibody diagnosis is performed by indirect fluorescent antibody test, competitive enzyme-linked immunosorbent assay (cELISA), and complement fixation test [
1]. Because of the reliability of the test and the convenience of mass testing, antigen diagnosis by PCR and antibody diagnosis by cELISA are mainly used [
2]. EP in the Republic of Korea (ROK) was 0.9%
T. equi-positivity from three provinces during 2007-2010 [
3,
4]. Climate change is having a significant impact on the increase in ticks and tick-borne diseases [
2]. There is a need to investigate changes in the infection status of EP according to Korean Peninsula warming. In this study, we monitored the EP infectious status in ROK during 2016-2017, using PCR for antigen detection and cELISA for antibody detection. Subsequently, phylogenetic analysis was used to compare the genetic relationships.
Horse blood and serum samples were collected as a part of the health management program by Korean Racing Authority. This article does not have any studies with human participants or animals done by any authors. We surveyed EP antigen and antibody in blood and serum samples collected from 1,650 horses at 222 horse ranches in 6 metropolitan cities and 9 provinces in ROK (
Table 1). The collected samples were stored at 4┬░C until used. Blood samples were tested for antigens of
B. caballi and
T. equi using PCR. DNA was extracted from the blood samples using a Maxwell RSC Whole Blood DNA kit (Promega, USA). DNA was eluted in 50 ┬ĄL volumes of elution buffer and stored at -70┬░C until used. The PCR reactions included 2 ┬ĄL of extracted DNA and 10 pmol of speci’¼üc primer sets (
Table 1) [
6], and run on a C1000 Touch
TM Thermal Cycler (Bio-Rad, USA) (95Ōäā for 10 minutes followed by 35 cycles at 95Ōäā for 1 minute, 60Ōäā for 1 minute, and 72Ōäā for 1 minute; and finally 72Ōäā for 5 minutes). PCR-positive samples were sequenced using Macrogen sequencing service (ROK). Nucleotide sequence homology searches were analyzed by the National Center for Biotechnology Information (NCBI) BLAST network service and aligned using the MegAlign software package (Windows version 7.1; DNA-STAR, USA). Phylogenetic trees were generated using neighbor-joining algorithms and the Jukes and Cantor matrix. Support for topology was calculated using 1,000 bootstrap replications. Serum samples were tested for anti-
B. caballi and anti-
T. equi antibodies by cELISA using
B. caballi and
T. equi antibody test kits (VMRD, USA), respectively. cELISA tests were performed according to the manufacturerŌĆÖs instructions. Briefly, 50 ╬╝L of serum samples and controls were loaded into the antigen-coated plate. After incubation, 50 ╬╝L of primary antibody was added to each well. And 50 ╬╝L of secondary antibody-peroxidase conjugate was added to each well. Finally, substrate solution and stop solution was consecutively added to each well and the ELISA plates were read on its optical density. Calculation of the percent inhibition (%I) was as follows:
%I (Inhibition rate) = 100 [1 - (sample optical density [OD]/negative control OD)]
The ELISA positive serum (n = 1) was collected at a total of three times including after 4 months and 1 year later. Ticks were collected from the
T. equi-positive horse ranch surrounding. Tick drag (BioQuip Products Inc.; USA) was used to collect ticks. Ticks were removed from drags, and transported to the laboratory where they were stored at -20┬░C until identification by microscopic morphology on a cold table using standard keys [
7].
During 2016-2017 in ROK, one of 1,650 horses (0.06%) was confirmed
T. equi antigen and antibody (
Table 2). In addition,
Theileria spp. was found in 2 of the 1,650 horses (0.12%), which showed seronegative for
T. equi and
B. caballi antibody test. We collected a total of 62 ticks in
T. equi-positive horse ranch, which were 52 ticks (2 nymphs and 50 larvae) in 2016 and 10 ticks (10 larvae) in 2017. All of them were
Haemaphysalis longicornis, in which EP pathogens were not detected.
The antibody titer of T. equi-positive horse at the first test was 54.63 %I. The second and third test of it were performed at 4 months and 1 year later, in which T. equi-antibody titers were showed 56.79 %I and 67.33 %I, respectively. At that time, we have also tested for 5 co-breeding horses with T. equi-positive horse in Ulsan at the same time to bleed T. equi-positive horse. No antibodies of T. equi or B. caballi were found in the other 5 co-breeding horses.
Three of 1,650 horses (0.18%) were
Babesia/Theileria duplex PCR-positive, which were from Ulsan, Gyeonggi-do, and Gyeongsangbuk-do, respectively (
Tables 1 and
2). Of them,
T. equi PCR-positive sample (17H107) was from Ulsan, in which
T. equi antibody was also detected (
Table 2,
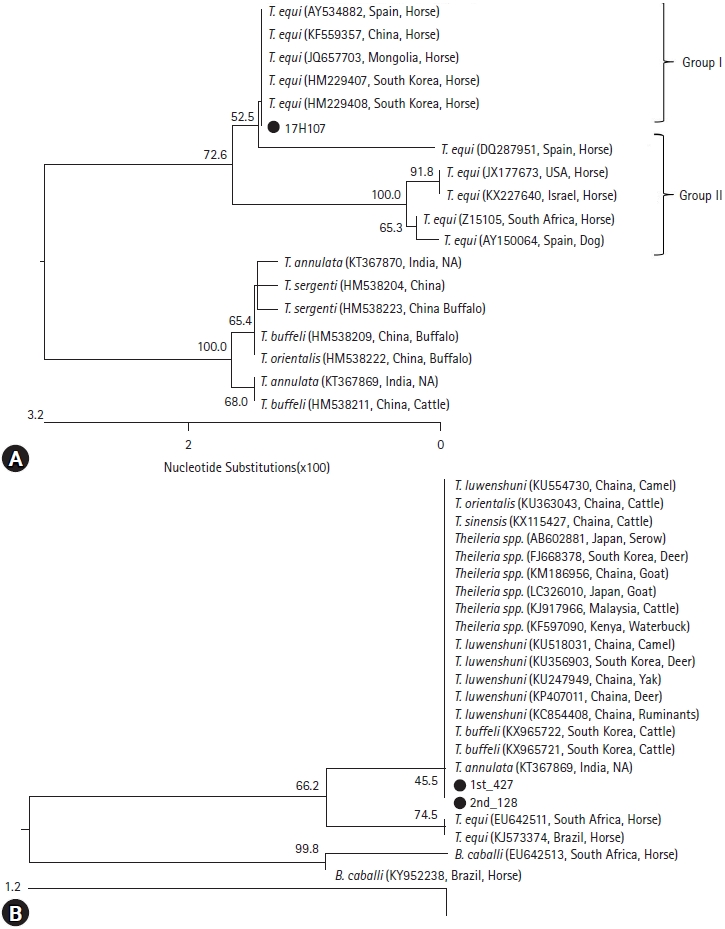
Fig. 1A). The 18S rRNA gene from 17H107 exhibited the highest genetic homology with two strains in ROK (HM229407, HM229408) (
Fig. 1A). Compared with those of other countries, 17H107 showed highly homologous with the strains in Chinese (KF559357) and Spanish (AY534882) horse blood and the strain in Mongolian horse ticks (JQ657703) (
Fig. 1A).
Two
Theileria spp., 1st _427 and 2nd _128, were also detected (
Fig. 1B). The
Theileria spp. were similar to
T. buffeli derived from cattle and
T. luwenshuni derived from deer in ROK. And, those were highly homologous with
T. luwenshuni,
T. orientalis, and
T. sinensis from China and
T. annulata from India (
Fig. 1B). However, two horses confirmed with
Theileria spp. showed no clinical signs.
T. equi was found in one horse in Ulsan (35┬░32'60"N, 129┬░18'60"E) in ROK during 2016-2017 at national level surveillance (
Table 2). Previously, three province surveillance of EP pathogens in Korea had been performed during 2007-2010 [
3]. In this study,
T. equi was detected in Ulsan, while
T. equi was found in Gyeonggi-do during 2007-2010 in the previous study (
Table 2). The positive regions between two studies was not related epidemiologically each other. There are 15 tick species that transmit
B. caballi - 7
Dermacentor spp., 6
Hyalomma spp., and 2
Rhipicephalus spp. [
1]. And there are 14
T. equi-transmissible tick species, which are 4
Dermacentor spp., 4
Hyalomma spp., 5
Rhipicephalus spp., and
Amblyomma cajennense [
1]. To date, above EP-transmissible tick species have not been found in horses and horse ranches in ROK [
8,
9]. The collected ticks around the
T. equi-positive horse ranch in this study were
H. longicornis, and no EP pathogens were detected in the ticks. In 2017, the positive rate (0.06%) of
T. equi was decreased, compared with that (0.9%) in 2010. This result might be due to absence of the transmissible tick species of EP pathogens in ROK and no transport of
T. equi-positive horses. However, the artificial infection experiments have reported that the propagation of
T. equi by
H. longicornis is possible [
10]. Therefore, it is needed consecutively to examine EP pathogens in horse ticks.
In other countries, seroprevalences of
T. equi in horses were from 78.8% to 0.0% depending on its infection and sanitation status. Sudan has the highest seroprevalence at 78.8%, followed by Mongolia (72.8%), Brazil (34.8%), China (3.8%), and Japan (0.0%) [
11-
15]. However, no
T. equi antibody-positive cases were found in Japan [
12]. The seroprevalence (0.06%) of
T. equi in ROK was almost ŌĆ£EP-freeŌĆØ status. Even though
B. caballi-positive horse was not found until now in Korea, the seroprevalence of
B. caballi was also different from each country. The seroprevalence of
B. caballi was the highest in Mongolia at 40.1%, followed by Brazil (27.2%), China (20.1%), Japan (7%), and Sudan (5.1%) [
11-
14]. To sustain ŌĆ£EP-freeŌĆØ status in ROK, thorough border quarantine inspection should be conducted continuously and the monitoring of EP for domesticated horses should be performed consecutively.
T. equi from a horse in Ulsan (17H107) exhibited the highest genetic homology with two
T. equi in ROK (HM229407, HM229408) through phylogenetic analysis of 18S rRNA gene (
Fig. 1A). Therefore,
T. equi in this study was confirmed to be the same genotype as the previously detected Korean strain (
Fig. 1A). Internationally, 17H107 showed highly homologous with the strains in Chinese (KF559357) and Spanish (AY534882) horse blood and the strain in Mongolian horse ticks (JQ657703) (
Fig. 1A). In USA, EP was confirmed in horses imported illegally from Mexico in 2008 [
5], indicating the importance of EP prevention by thorough border quarantine. It is important to quarantine animals at national border for sustaining national-free status.
Two
Theileria spp. was detected in 2 horses (
Fig. 1B), which showed the genetic similarity with 4
Theileria spp., such as
T. buffeli,
T. luwenshuni,
T. orientalis,
T. sinensis, and
T. annulate (
Fig. 1B). However, those have never been observed to infect horses in any previous report. While
T. luwenshuni and
T. orientalis have been reported in infected water deer and cattle in the ROK, respectively [
16,
17], there are no reports of
T. sinensis and
T. annulata among 4
Theileria spp. in ROK. Additionally, two horses confirmed with
Theileria spp. showed no clinical signs. Thus, two
Theileria spp. might be non-pathogenic.
In conclusion, EP by T. equi was found in ROK during 2016-2017, showing 0.06% T. equi-positivity. It is still low prevalence, compared with that of during 2007-2010. Furthermore, there was no T. equi-transmissible tick species in surrounding of T. equi-positive ranch. However, the predominant tick species in horse, H. longicornis, can transmit T. equi experimentally. Therefore, the monitoring of EP pathogens in horses and ticks will be useful for accomplishing EP-free status in ROK.















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print