Immune-mediated hemolysis after administration of human intravenous immunoglobulin in a dog: a case report
Article information
Abstract
A 10-year-old spayed female Maltese presented with purpura and hematemesis. Initial laboratory evaluation revealed immune-mediated thrombocytopenia, but evidence of hemolytic anemia was not identified. Three milligrams of human intravenous immunoglobulin (hIVIG) was administered for 3 hours following prednisolone and mycophenolate mofetil. A pale mucous membrane was identified, and the packed cell volume decreased by 3%. Blood film examination revealed significant spherocytosis with auto-agglutination. Blood transfusions and immunosuppression were continued for 4 days, and hIVIG was discontinued. This report describes a case of increased immune-mediated hemolysis after hIVIG administration, possibly due to new-onset immune-mediated hemolytic anemia or enhanced immunogenicity.
Human intravenous immunoglobulin (hIVIG) is a purified and concentrated form derived from human plasma. It contains more than 90% intact, biologically active immunoglobulin (Ig) G, along with trace amounts of IgA, IgM, CD4, CD8, and human leukocyte antigen molecules [1]. hIVIG is used to provide passive immunity to individuals with immune deficiencies and as an immunomodulator of immune-mediated diseases in humans [2]. Despite no consensus or definitive indication in veterinary medicine, there have been reports of hIVIG use in immune-mediated diseases in animals [3].
hIVIG is generally well tolerated in humans, with adverse events occurring in less than 5%. Common adverse effects include fever, chills, and headache. Rare, but potentially serious adverse effects, include aseptic meningitis, kidney injury, hypersensitivity reactions, and thrombosis. hIVIG-induced hemolysis is a potentially fatal but rare adverse effect. The main mechanism in humans is the presence of blood group antibodies (anti-A and anti-B) in the IVIG products. Risk factors include high cumulative doses (> 2 g/kg), positive direct antiglobulin test (DAT) results, underlying inflammatory conditions, and non-O blood types, particularly types A and AB [4]. To our knowledge, only one case of suspected hIVIG-induced hemolysis has been reported in veterinary medicine [5]. Here, we report a case of immune-mediated hemolysis in a dog after the administration of hIVIG.
A 10-year-old spayed female Maltese presented with a one-week history of purpura and thrombocytopenia. Prednisolone (PDS) 1.5 mg/kg/day and mycophenolate mofetil (MMF) 40 mg/kg/day were intermittently administered for thrombocytopenia suspected to be caused by immune-mediated thrombocytopenia (ITP) for up to 3 days before referral. However, the symptoms of purpura, collapse, hematemesis, anemia, and thrombocytopenia worsened severely following treatment. The dog did not receive either blood transfusion or hIVIG previously.
Physical examination revealed the dog had multifocal petechiae, purpura (Fig. 1), and pale pink mucous membranes. Manual packed cell volume (mPCV) measurements indicated severe anemia, with a value of 19% and 6.5 g/dL of total plasma protein. A complete blood count revealed macrocytic normochromic severe anemia (hematocrit, 17.2%; mean corpuscular volume, 76.8 fL; mean corpuscular hemoglobin concentration, 32.6 g/dL) and evidence of reticulocyte production (reticulocyte count, 327.5 × 103/μL). The dog also showed severe thrombocytopenia on an automated cell counter (platelet count, 0 × 109/L) (Table 1). There were only 4 spherocytes per × 1,000 oil immersion fields and no auto-agglutination on the saline agglutination test (SAT) after mixing 4 drops of saline with one drop of blood (Fig. 2A and C). A tick/vector comprehensive real-time polymerase chain reaction panel (IDEXX, USA) for Mycoplasma haemocanis, Babesia spp., Ehrlichia spp., Anaplasma spp., Rickettsia spp., and Hepatozoon spp, was negative. Serum biochemistry showed mild hyperglycemia (149 mg/dL; reference range, 70–143 mg/dL) and elevated symmetric dimethylarginine (16 μg/dL; reference range, 0–14 μg/dL). Urinalysis of voided urine revealed hemoglobinuria (50 mg/dL, 3+). The exclusion of toxins and drugs was accomplished through history-taking. Therefore, idiopathic ITP was tentatively diagnosed. However, there is no evidence of concurrent immune-mediated hemolytic anemia (IMHA), based on the consensus statement [6].
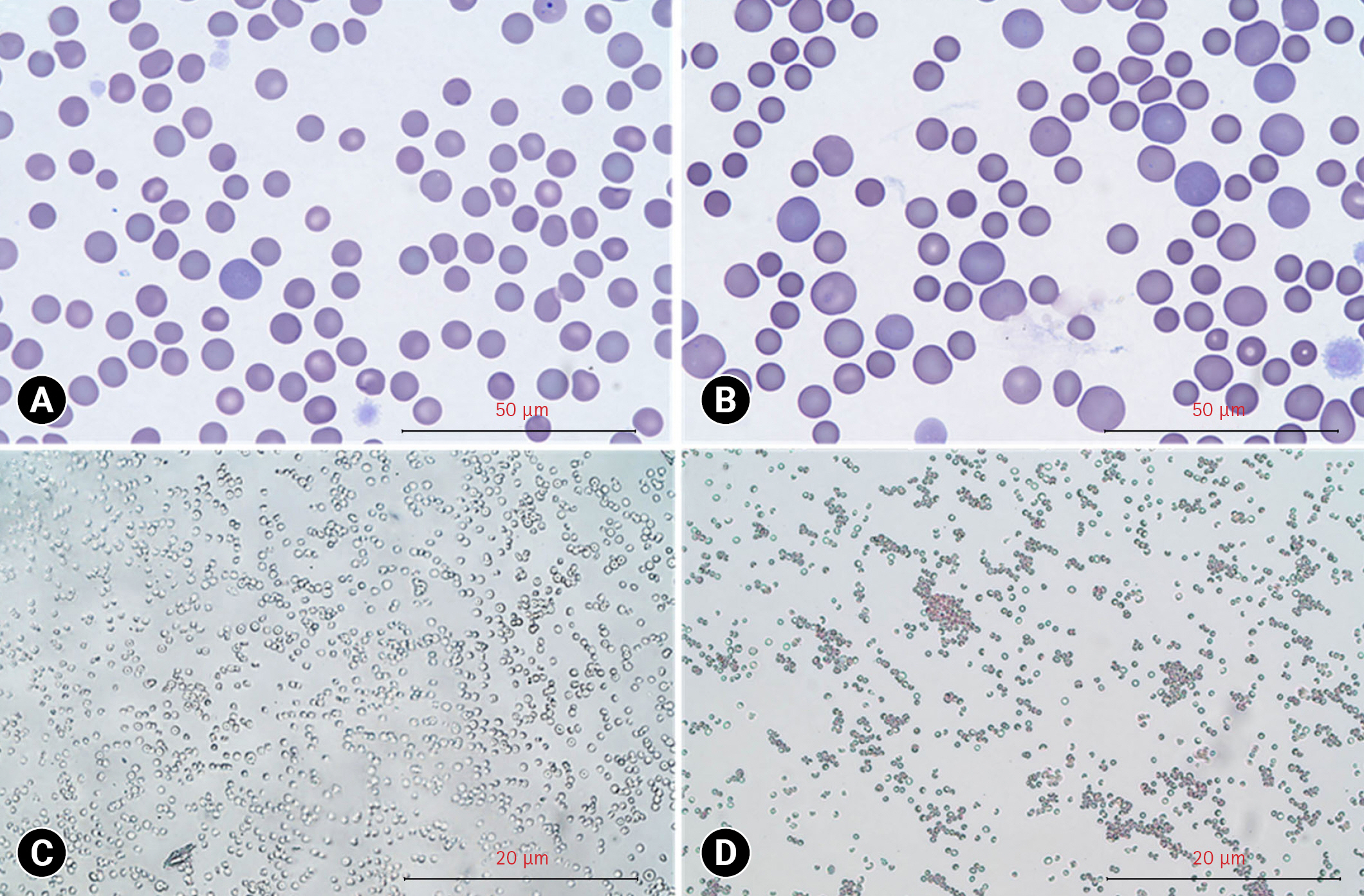
Changes of red blood cell morphology and agglutination after human intravenous immunoglobulin (hIVIG) administration. Red blood cell morphology before (A) and after (B) hIVIG administration (Diff-Quick stain, scale bar = 50 μm). Saline agglutination test results in negative before (C) and after (D) hIVIG administration (scale bar = 20 μm).
As treatment for ITP, PDS (2 mg/kg/day) and MMF (10 mg/kg/day) were administered orally, and vincristine (0.02 mg/kg, once) was administered intravenously. For immune modulation, subsequent 0.6 mg/kg hIVIG was administered. To prevent an anaphylactic reaction, 0.2 mg/kg of chlorpheniramine was administered before hIVIG administration. The continuous infusion of hIVIG was performed over a period of 3 hours.
A pale mucous membrane was observed 3 hours after the completion of hIVIG administration. The mPCV decreased from 19% to 16%. SAT was performed by mixing 4 drops of saline with one drop of blood to confirm agglutination. Remarkable spherocytosis was observed with 13 spherocytes per × 1,000 oil immersion field (Fig. 2B. According to the American College of Veterinary Internal Medicine consensus statement on the diagnosis of IMHA, these findings support the immune-mediated destruction of red blood cells [6]. DAT results using immunochromatographic strips were negative.
Packed red blood cell transfusions were also given, and no other acute or chronic side effects were identified. Until discharge, spherocytes were < 5 per ×1,000 oil immersion field, the mPCV was maintained at 35% to 40%, and the platelet count gradually increased over 4 days of hospitalization.
This report describes a rare case of IMHA in a dog after the administration of hIVIG. Only one case of suspected hIVIG-induced hemolysis has been reported [5], which involved a two-year-old dog diagnosed with pemphigus foliaceus that received 2 consecutive doses of hIVIG at 0.5 g/kg/day. Hemolytic anemia developed on the third day after the first dose, but evidence for immune-mediated hemolysis was lacking. However, obvious evidence of IMHA was seen in this case, thus caution is needed when administering hIVIG as an immunosuppressant for immune-mediated diseases in dogs.
Based on the history and laboratory values, the cause of anemia identified prior to hIVIG administration was thought to be gastrointestinal blood loss secondary to ITP. However, it is not possible to completely exclude the possibility of Evans syndrome because of the history of immunosuppressive therapy for a short period, only 4 spherocytes per × 1,000 oil immersion fields, and hemoglobinuria. However, even if IMHA is the underlying disease, the administration of hIVIG has been shown to enhance the immunogenicity of anti-red blood cell antibodies, as indicated by the significant increase in the number of spherocytes and positive SAT results. While the ABO antigen is the primary cause of hIVIG-induced hemolysis in humans, other factors, such as a high hemolysin titer, specific excipients used in the IVIG formulation, highly human leukocyte antigen-sensitized red blood cells, and CD16 polymorphism in macrophages, can contribute to hIVIG-induced hemolysis [5]. The transfusion of blood products between different species can lead to significant compatibility issues [7,8]. Although dogs and humans share similarities in their blood components, important differences can trigger immune responses and potentially result in severe adverse reactions or even death. A study evaluating cross-match tests between dog and human blood found that when human plasma was exposed to dog blood cells, hemolysis or agglutination occurred in all samples, regardless of the blood type. The frequency of hemolysis was higher than that of agglutination, suggesting that human plasma is incompatible with canines and that the transfusion of human whole blood or plasma carries a high risk of transfusion reactions if administered to dogs [9].
Since 2011, the United States Food and Drug Administration has reported an increase in hemolytic transfusion reactions following hIVIG treatment. The IVIG Hemolysis Pharmacovigilance Group has defined IVIG-associated hemolysis as a hemolytic episode that occurs within 10 days of IVIG administration, accompanied by a decrease in hemoglobin of less than 10 g/L and a positive DAT, along with at least 2 of the following criteria: elevated reticulocyte count; elevated serum lactate dehydrogenase; elevated serum unconjugated bilirubin; low serum haptoglobin; presence of hemoglobinemia; presence of hemoglobinuria; or evidence of spherocytosis. The exclusion criteria are the presence of other causes of anemia, negative DAT results, and no hemolysis [10]. In this case, the exacerbation of hemolytic anemia, evidence of spherocytosis, and positive SAT after hIVIG administration support the diagnosis of IVIG-associated hemolysis.
Despite positive SAT results, the DAT was negative in this case. Although the DAT result is the first criterion for classifying hIVIG-induced hemolytic anemia, there are differences in the use and interpretation of DAT between human and veterinary medicine, which raises questions about the applicability of the human criteria of hIVIG-induced hemolysis in veterinary medicine. When performed correctly and in an appropriate clinical context, DAT has shown a positive predictive value of 97% to 99% in human medicine. However, veterinary clinicians have raised concerns about the possibility of false-negative or false-positive DAT results. False-negative and false-positive DAT results in veterinary medicine could be attributed to various factors, including ineffective reagents, faulty methods, incorrect test interpretation, and interference from treatments such as immunosuppressive drugs and transfusions [6]. The sensitivity of DAT in dogs has been reported to range from 48% to 82% [11–14], indicating that relying solely on DAT for diagnosing IMHA may lead to missed diagnoses. In veterinary medicine, SAT, which involves mixing 4 drops of saline with one drop of blood, has been reported to have a sensitivity of 88% for agglutination detection [15], which is similar to the sensitivity of DAT. Therefore, both DAT and SAT results should be considered when evaluating hIVIG-induced hemolysis in veterinary medicine. Both methods demonstrated specificity values of 94% to 100%, indicating that immune-mediated destruction can be inferred if a positive result is confirmed by either method. Therefore, based on the positive SAT result confirmed after hIVIG administration in this case, it can be considered that immune-mediated hemolysis occurred. Considering the limitations of using DAT as the sole criterion for excluding hIVIG-induced hemolysis in veterinary medicine, further research is needed to establish criteria specific to veterinary medicine.
This report has described a case of increased immune-mediated hemolysis as an adverse event after the administration of hIVIG, which may have been due to new-onset IMHA or the enhanced immunogenicity of pre-existing IMHA. Clinicians should always consider the risk of immune-mediated hemolysis as an adverse effect when administering hIVIG as an immunosuppressant in immune-mediated diseases.
Notes
The authors declare no conflict of interest.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2020R1C1C1008675).


