Assessment of prognostic factors in dogs with mammary gland tumors: 60 cases (2014-2020)
Article information
Abstract
Canine mammary gland tumors are the most common neoplasms in intact female dogs. Approximately half of all mammary tumors are malignant, and there is a risk of metastasis, which is associated with a poor prognosis. This study was to evaluate the prognostic factors of canine mammary gland tumors and the risk factors associated with the development of malignant tumors. From 2014 to 2020, 60 dogs with mammary gland tumors that underwent surgical treatment were evaluated in this retrospective study. Tumor size, TNM stage, and histopathological results were prognostic factors for 2-year survival after surgery. Every 10 mm increase in tumor size, increased the risk of death within 2 years after surgery 1.213 times. Dogs with TNM stage IV or V had 8.667 fold risk of death within 2 years after surgery. The 2-year survival rate for dogs with benign tumors was 90.2% and for malignant tumors was 67.3%. Tumor size is the most important prognostic factor for canine mammary gland tumors. As tumor size increased by 10 mm, the risk for development of malignant tumors increased by 1.487 times. Tumors larger than 30 mm are highly likely to be malignant, and metastatic evaluation and wide resection should be considered.
Introduction
Mammary gland tumors (MGTs) are the most common neoplasm in intact female dogs that account for over 40% of all tumors [1,2]. Surgical resection remains the standard treatment for most types of MGTs, except in cases of inoperable highly metastatic disease and most inflammatory mammary carcinomas [1,3,4]. Surgical efficacy and feasibility depend on the number of tumors, tumor size, location, and patient status [5]. Dogs in early stages of tumor progression, according to the TNM classification of malignant tumors, and small tumors are often treated effectively with surgery alone. Dogs with benign MGTs and approximately 50% of dogs with malignant MGTs are also treated with surgery alone [6,7].
The risk factors that influence the prognosis of dogs with MGTs include age, ovariohysterectomy (OHE) at the time of tumor resection, tumor size, TNM stage, and tumor histopathological type. Increased age has been associated with lower 2-year survival rates in dogs with MGTs [8]. It has been reported that there is no prolonged survival time in dogs with OHE at the time of tumor resection, compared with dogs that were not spayed [9-11]; however, dogs treated with OHE at the time of tumor resection lived significantly longer than dogs treated with tumor removal alone [12]. Tumor size is one of the most important risk factors associated with the prognosis of dogs with MGTs [13-16]. Dogs with a low-level TNM stage lived significantly longer than dogs in late stage [15-18]. Metastasis to regional lymph nodes, distant metastasis, and TNM stage are strongly associated. Approximately 35% to 50% of canine MGTs are malignant [4] and most malignant tumors eventually metastasize [19]. The likelihood of metastasis increases when the tumor is malignant [17,20].
Preoperative evaluation of prognosis provides the veterinarian with important information that may influence treatment planning. The purpose of this study was to evaluate the prognostic factors of MGTs in dogs and to suggest appropriate guidelines for treatment.
Materials and Methods
Study population and inclusion criteria
The medical records of dogs with MGTs that received surgical treatment at the Chungnam National University Veterinary Medical Teaching Hospital from 2014 to 2020 were reviewed and included in this study. Dogs without preoperative examination results, histological examination results, or follow-up data were excluded from this study. Data obtained from the medical records, including clinical data such as signalment, blood analysis results (complete blood count, serum biochemical profile), and cytological examination results (fine needle aspiration), were included. Tumor number, size, and location were also included. Tumor size was decided as the maximum diameter of the tumor among all of the MGTs for each dog. Thoracic radiography and standard abdominal ultrasonography were performed. Computed tomography was performed in patients with malignant tumors. Tumor resection was performed based on the diagnostic examination, and surgical techniques (lumpectomy, simple mastectomy, regional mastectomy, unilateral mastectomy or total mastectomy) were recorded. Histopathological examination was performed after the tumor resection. The clinical stage of the MGTs was assigned according to a modified version of the World Health Organization TNM staging classification [3,21].
Follow-up examination
Follow-up examinations, including physical examination and imaging procedures (thoracic radiography and abdominal sonography), were performed every 3 months after surgery. When follow-up examinations were not possible, data were obtained through telephone interviews with the owners. Follow-up data included local relapse, distant metastasis, and MGT-related death. The dogs died by metastasis, rucurrence or euthanatized for MGT was defined as MGT-related death. In dogs that died of causes unrelated to the MGTs, death was defined as censored data for calculating survival time.
Statistical analysis
The data obtained included sex, age, body weight, number of tumors, tumor size, TNM stage, histopathological results, surgical procedures, ovario-uterine disease, relapse, and death. Overall survival (OS) and disease-free period (DFP) were evaluated. OS was calculated from the date of surgical removal of the tumor to the date of death or the last follow-up date. The DFP was defined as the period from tumor resection to relapse. Kaplan-Meier survival curve analysis was used to create category-specific survival curves. The Cox proportional hazards model was used to estimate the effect of each factor (sex, age, body weight, the number of tumors, tumor size, lymph node involvement, distant metastasis, TNM stage, histopathological results, surgical procedures, ovario-uterine disease, and OHE with tumor resection) potentially associated with survival time after tumor resection. The Mann-Whitney test was used to investigate the differences between benign and malignant tumors depending on their size. Logistic regression was used to estimate the risk factors (sex, age, body weight, number of tumors, tumor size, and ovario-uterine disease) associated with malignant MGTs. All statistical analyzes were performed using commercial statistical software (IBM SPSS Statistics ver. 24.0; IBM Corp., USA).
Results
In this study, 69 dogs underwent tumor resection for the surgical treatment of MGTs. Of those, 7 dogs that had no histological examination and 2 dogs that had no follow-up data were excluded. A total of 60 dogs that received surgical treatment for MGTs between 2014 and 2020 met the criteria for inclusion in the study.
The breed distribution was as follows: Beagle (n = 1), Chihuahua (n = 1), Cocker Spaniel (n = 4), Jindo (n = 2), Maltese (n = 15), miniature Pincher (n = 1), miniature Schnauzer (n = 2), mixed (n = 8), papillon (n = 1), Pomeranian (n = 2), Poodle (n = 9), Shih Tzu (n = 7), Spitz (n = 1), Welsh Corgi (n = 1), and Yorkshire Terrier (n = 5).
Of a total of 60 dogs, 51 dogs (85.0%, 51/60) were intact females and 9 dogs (15.0%, 9/60) were spayed females. There were 36 dogs (60.0%, 36/60) with benign tumors (complex adenoma [n = 16], adenoma [n = 10], and benign mixed tumor [n = 10]) and 24 dogs (40.0%, 24/60) with malignant tumors (adenocarcinoma [n = 13], carcinoma [n = 9], extraskeletal osteosarcoma [n = 1], and soft tissue sarcoma [n = 1]). Of the intact female dogs, 32 had benign tumors and 19 had malignant tumors. Of the spayed female dogs, 4 dogs had benign tumors and 5 dogs had malignant tumors.
The incidences of benign and malignant tumors are summarized in Table 1. The mean age of the subjects with benign tumors was 10.9 ± 3.1 years (median, 11.0; range, 3.0-18.0 years) and mean body weight was 5.4 ± 4.2 kg (median, 3.8; range, 2.1-24.0 kg). The mean age for malignant tumors was 11.7 ± 2.9 years (median, 12.0; range, 2.0-16.0 years) and mean body weight was 5.1 ± 2.6 kg (median, 4.6; range, 2.0-11.0 kg).
In this study, there was a significant difference between tumor size and histopathological tumor type (p = 0.008). The mean size ± standard deviation of benign tumors was 20.0 ± 15.2 mm (median, 15.5 mm; range, 3-60 mm) and for malignant tumors, 48.7 ± 40.2 mm (median, 37.5 mm; range, 7-141 mm). Tumor size was correlated with malignancy (p = 0.002).
As tumor size increased by 10 mm, the risk of developing malignant tumors increased 1.487 times (p = 0.004). Tumors larger than 50 mm had an 11.815–fold risk of developing into malignant tumors compared to tumors less than 30 mm (p = 0.001).
Tumor size, malignancy and high-level TNM stage were significantly associated with 2-year survival after tumor resection (Table 2). Every 10 mm increase in tumor size, increased the risk of death within 2 years after tumor resection 1.213 times (p = 0.011). Dogs with tumors larger than 50 mm had a 5.448 higher risk of death within 2 years after tumor resection compared to those with tumors less than 30 mm (p = 0.021). In dog with the presence of lymph node involvement, the risk of death within 2 years after tumor resection increased by 8.667 times (p = 0.001) compared to those with no lymp node involvement, whereas in dogs with the presence of distant metastasis, the risk was increased 72.665 times (p = 0.003) compared to those with no distant metastasis. Dogs with high-level TNM stage (IV or V) were 8.667 times at risk of death within 2 years after tumor resection compared to those with low-level TNM stage (I, II, or III).
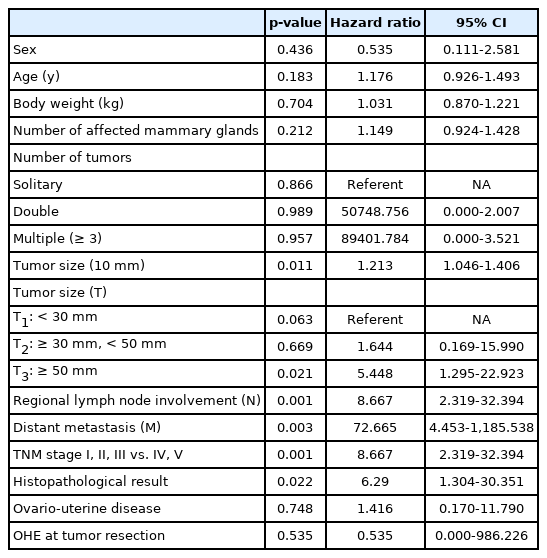
Prognostic factors associated with 2-year survival after surgery in 60 dogs with mammary gland tumors
There was a significant difference in the 2-year survival rate between dogs with low-level TNM stage and high-level TNM stage (p < 0.001). The 2-year survival rate for dogs with low-level TNM stage was 87.8%, and 33.3% for dogs with high-level TNM stage (Fig. 1A). Mean survival time of dogs with low-level TNM stage was 22.2 ± 0.8 months, but for those with a progressed TNM stage was 11.2 ± 3.6 months.
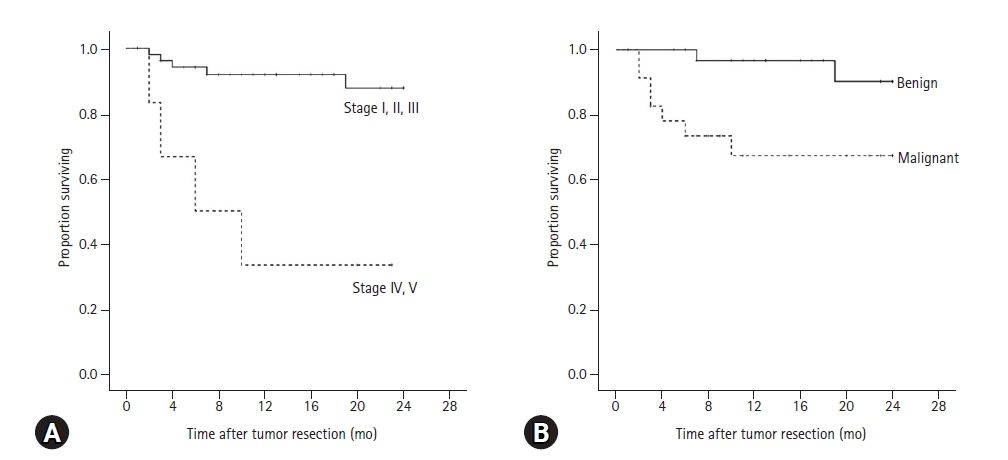
Kaplan-Meier analysis for 2-year survival rate of 60 dogs with mammary gland tumors after surgery according to (A) clinical TNM stage and (B) histopathological results. (A) The 2-year survival rate for dogs with low TNM stage was 87.8%, and 33.3% (p < 0.001) for dogs with high-level TNM stage. Mean survival time of dogs with low-level TNM stage was 22.2 ± 0.8 months, but for those with a high-level TNM stage was 11.2 ± 3.6 months. (B) The 2-year survival rate for dogs with benign tumors was 90.2% and for malignant tumors was 67.3% (p = 0.008). Mean survival of dogs with benign tumors was 23.1 ± 0.6 months, but 17.7 ± 2.0 months for dogs with malignant tumors.
The 2-year survival rate for dogs with benign tumors was 90.2% and for malignant tumors was 67.3% (Fig. 1B). Mean survival of dogs with benign tumors was 23.1 ± 0.6 months, but 17.7 ± 2.0 months for dogs with malignant tumors. There was a significant difference in the 2-year survival rate after tumor resection between benign and malignant tumors (p = 0.008).
Of the total of 60 dogs, 17 dogs (28.3%, 17/60) died within 2 years after tumor resection. Of the 17 dogs, 8 had benign tumors. One dog with a benign tumor died due to the occurrence of inflammatory carcinoma. Another dog died during chemotherapy because of tumor relapse and lymph node metastasis. Six dogs with benign tumors died from unrelated causes of MGTs. Nine dogs with malignant tumors died within 2 years, of which 7 died by MGTs and 2 died from unrelated causes.
Discussion
This study analyzed 60 cases of canine MGTs to evaluate the prognosis after tumor resection. Previous studies revealed that the prognostic factors of dogs with MGTs are age, tumor size, TNM stage, and OHE at the time of tumor resection [6,8-11,13-15,17-18, 22].
Approximately 35% to 50% of canine MGTs are malignant [6]. Similar to previous studies, the rate of dogs with malignant tumors was 40.0% (24/60) in the present study. One study reported that the risk for incidence of malignant tumors increases with increasing tumor size, in the study, the mean size of benign tumors was 21 mm and that of malignant tumors was 47 mm [20]. In this study, similar results were found that the mean size of benign tumors was 20 mm, but that of malignant tumors was 48.7 mm. In addition, there was a correlation between tumor size and the incidence of malignant tumors. Only 23.7% of tumors less than 30 mm were malignant, but 50.0% of tumors between 30 mm and 50 mm, and 78.6% of tumors larger than 50 mm were malignant.
There have been no reports on the quantity of tumors related to the incidence of malignant tumors. There was no significant correlation between the number of tumors and malignancy in the present study. However, 22.2% of solitary MGTs, 33.3% of double MGTs, and 45.2% of multiple MGTs were diagnosed as malignant in this study. Considering this point, the possibility of malignancy could not be ruled out even for a single MGT. This could be the basis for early wide resection rather than simple mastectomy or lumpectomy.
Several studies have revealed that inreased age, large tumor size, progressed TNM stage, and malignant tumors were related to poor prognosis [8,13-18,20]. Dogs with progressed TNM stage had shorter median survival time after tumor resection compared with dogs with clinical stage I, II, or III [17]. OHE at the time of tumor resection prolonged survival time in one study [12], but not in others [9-11].
According to several studies, the most important prognostic factor for canine MGTs is tumor size [13-16]. In this study, as the tumor size increased 10 mm, the risk of death within 2 years after surgery also increased 1.213 times (p = 0.011). In addition, tumor size was correlated with the development of malignant MGTs. The larger the tumor size, the higher the likelihood of malignancy and metastasis, which are considered to affect the 2-year survival rate after tumor resection.
Unlike tumor size, the side and location of affected glands have been reported to be unrelated to prognosis [17]. Likewise, in this study, the number of tumors and the number of affected mammary glands did not affect the incidence of malignant tumors or the 2-year survival rate after tumor resection. Our results suggest that only tumor size was a risk factor associated with the development of malignant MGTs.
Dogs with benign MGTs and approximately 50% of dogs with malignant MGTs are treated with surgery alone [6,7]. However, dogs that previously had MGTs have a risk of developing new tumors in other mammary glands. Because approximately 58% of dogs with MGTs relapse after surgical removal of benign tumors, regional mastectomy is recommended, even with single tumors suspected to be benign [23].
In our cases, 9 dogs died due to MGTs within 2 years after tumor resection: 2 dogs had benign tumors, and one of them died because of the occurrence of inflammatory carcinoma after surgery. Another dog was diagnosed with tumor relapse and lymph node metastasis and died during chemotherapy. Except for these 9 dogs, dogs with benign tumors or without metastasis had a good prognosis.
According to the results of present study, high-level TNM stage was closely related with the incidence of malignant tumors. The larger tumor size, the more likely it is to be the malignant tumor, and the malignant tumor have the risk of metastasis. Considering these points, tumor size is one of the most important factor associated with incidence of malignancy and 2-year survival after tumor resection. Therefore, surgical intervention in the early stages is considered to be helpful for increasing the 2-year survival rate.
In conclusion, the prognostic factors were tumor size and high-level TNM stage. Among these, tumor size could be an important criterion for the preoperative determination of malignant tumors. Because tumors larger than 30 mm are highly likely to be malignant, metastatic evaluation is required, and wide resection is recommended.
Notes
The authors declare no conflict of interest.
Acknowledgements
This work was supported by a research fund from the Chungnam National University.

